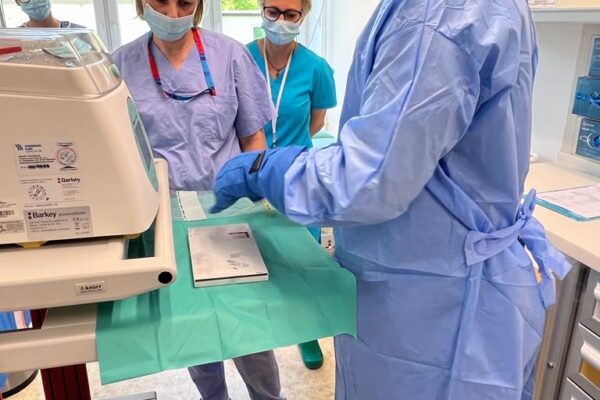
FIRST INFUSION OF CHIMERIC ANTIGEN RECEPTOR (CAR) T CELLS IN DEPARTMENT OF HAEMATOLOGY
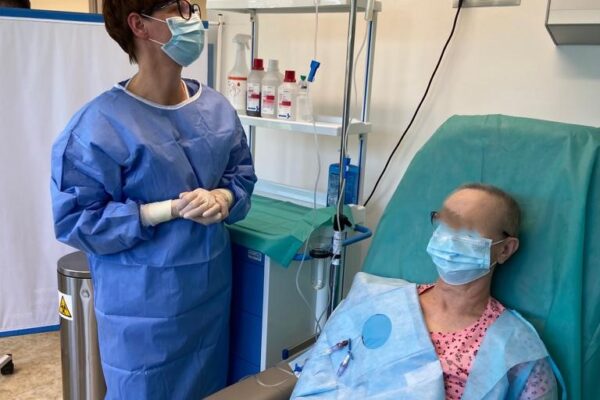
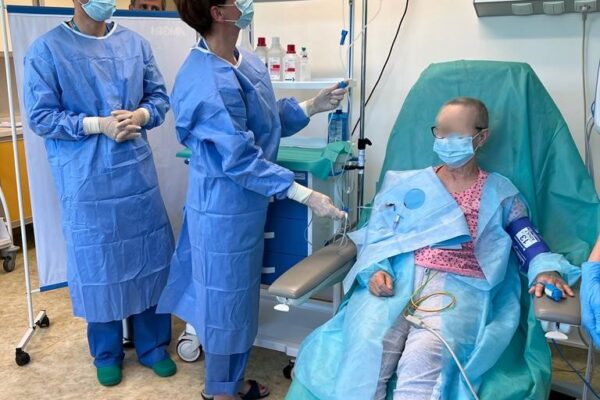
On May 29 an innovative therapy with chimeric antigen receptor-modified T (CAR-T) cells was used for the first time in the Department of Haematology Medical University of Lodz. A patient with diffuse large B-cell lymphoma appeared treatment-resistant and all the previous attempts with chemiotherapy proved ineffective. The only solution left was CAR-T cells infusion. The therapy outcomes will be assessed in the coming weeks.
Haematology and Transplantology Ward at the Department of Haematology in Lodz underwent a strict control and certification process by Novartis, the company which has the license for allogenic CAR-Ts therapy in relapsed or refractory DLBCL and refractory/relapsed acute lymphoblastic leukaemia in young patients.
CAR-T therapy is a global breakthrough in haematology. Unavailable to Polish patients several years ago (owing to its high cost: over 1 million per infusion), it has recently been reimbursed under the drug program of the National Health Fund.
It took the MUL Department 2 years to get ready for the therapy implementation. During that period, the quality control system was expanded, training was provided, and all the necessary licenses and permits were obtained from Poltransplant and National Centre for Tissue and Cell Banking. Moreover, Department of Genetic Engineering was established, and the necessary equipment was purchased for Apheresis and Cell Therapy Laboratory and the Bank of Hematopoietic Cells Bank. The MUL Department of Haematology is the 6th Polish centre which offers the CAR-T therapy to adult patients.
Implementation of CAR-T therapy opens new development possibilities for the MUL Department of Haematology and it will offer innovative genetic therapies for our patients in the future, says Prof. Agnieszka Wierzbowska, the Head of the Department. Currently, the MUL Department of Haematology is engaged in two clinical trials based on chimeric antigen receptor-modified T cells.
What is CAR-T?
CAR-T therapy is a modern and effective method of treating blood cancers which uses the patient’s own immune cells to defeat the disease. It involves genetically modified T cells with a chimeric antigen receptor which is able to locate and destroy neoplastic cells.
How does it work?
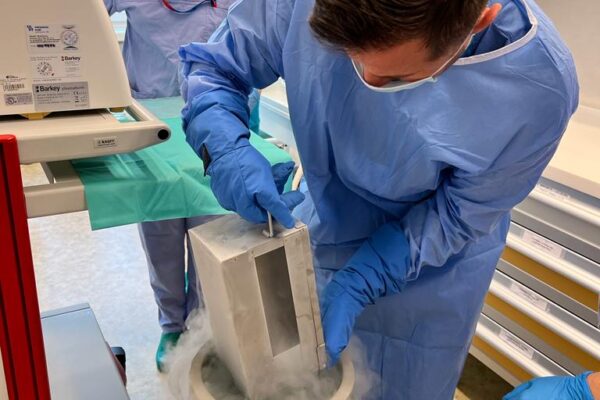
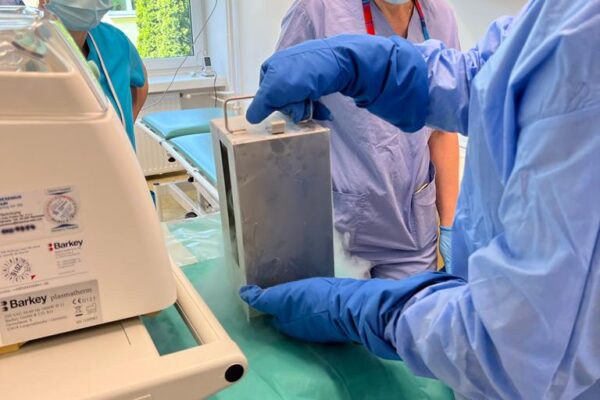
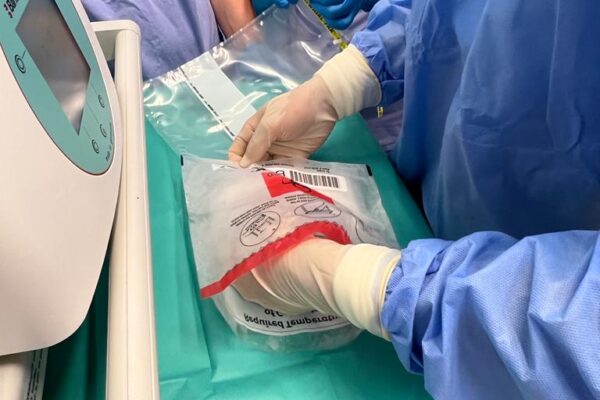
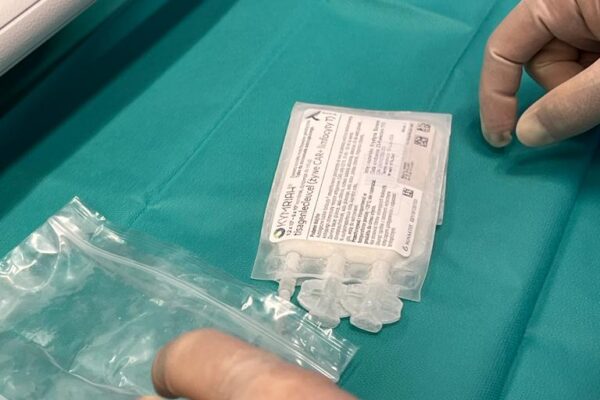
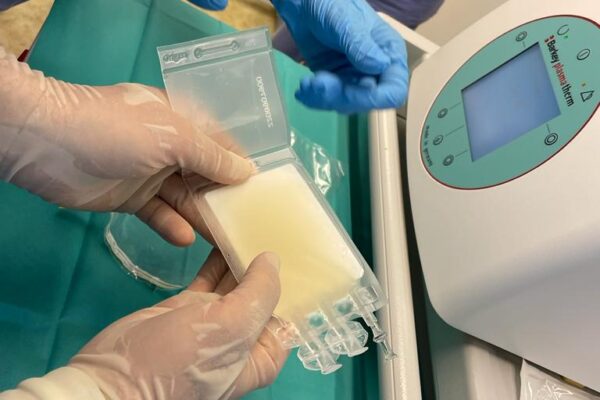
The chimeric antigen receptor is designed to recognise a specific antigen on the neoplastic cell. KYMRIAH is a CD19-directed autologous T cell immunotherapy. CAR-T cells preparation process involves the cells collection (leukapheresis) and transfer to a specialists genetic engineering laboratory cooperating with Novartis. Then a CD19-directed chimeric antigen receptor is infused into the patient’s T cells. Such genetically modified cells return to the Centre already as a specialist medication which is collected by Kopernik Hospital Genetic Engineering Department and stored in liquid nitrogen until the therapy is initiated. After the patient is adequately prepared, the CAR-Ts are defrosted and administered intravenously. Modified CAR-T cells act like an intelligent drug as they locate and destroy cancer cells. CAR-T therapy allows to achieve and prolong the disease remission, as well as offering an alternative for and multidrug chemotherapy and/or radiotherapy.